EMERALD study - a breakthrough in the treatment of ESR1-mutated ER+/HER2- advanced breast cancer
Last updated
Which endocrine therapy is most effective for ER+/HER2- mBC patients with an ESR1 mutation?
Breast cancer is the most common cancer among women. It is estimated that 2.3 million new cases of breast cancer are diagnosed globally each year. Over 80% of breast cancer (BC) patients have estrogen receptor-positive (ER+) tumors, which proliferate in response to estrogen. [1]
Endocrine therapy (ET), with either aromatase inhibitors (AI) or fulvestrant, plus a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor is the recommended first-line standard of care (SOC) for locally advanced or metastatic ER–positive/human epidermal growth factor receptor 2 (HER2)–negative breast cancer. [1]
Despite the efficacy of endocrine therapies, a large proportion of patients relapse due to acquired resistant disease. [2]
Over the last 10 years, there have been accumulating reports of ESR1-specific mutations leading to endocrine therapy resistance. These mutations rarely exist (0–3%) in primary tumors but are relatively common in metastatic endocrine therapy-resistant breast cancer lesions, with a wide-ranging prevalence of 6–55%. [3]
Elacestrant is a novel, nonsteroidal, oral SERD that degrades the ligand-binding domain of estrogen receptor gene α (ESR1) in a dose-dependent manner and has been shown in preclinical trials to inhibit tumor growth, including those harboring ESR1 mutations associated with endocrine resistance. [1]
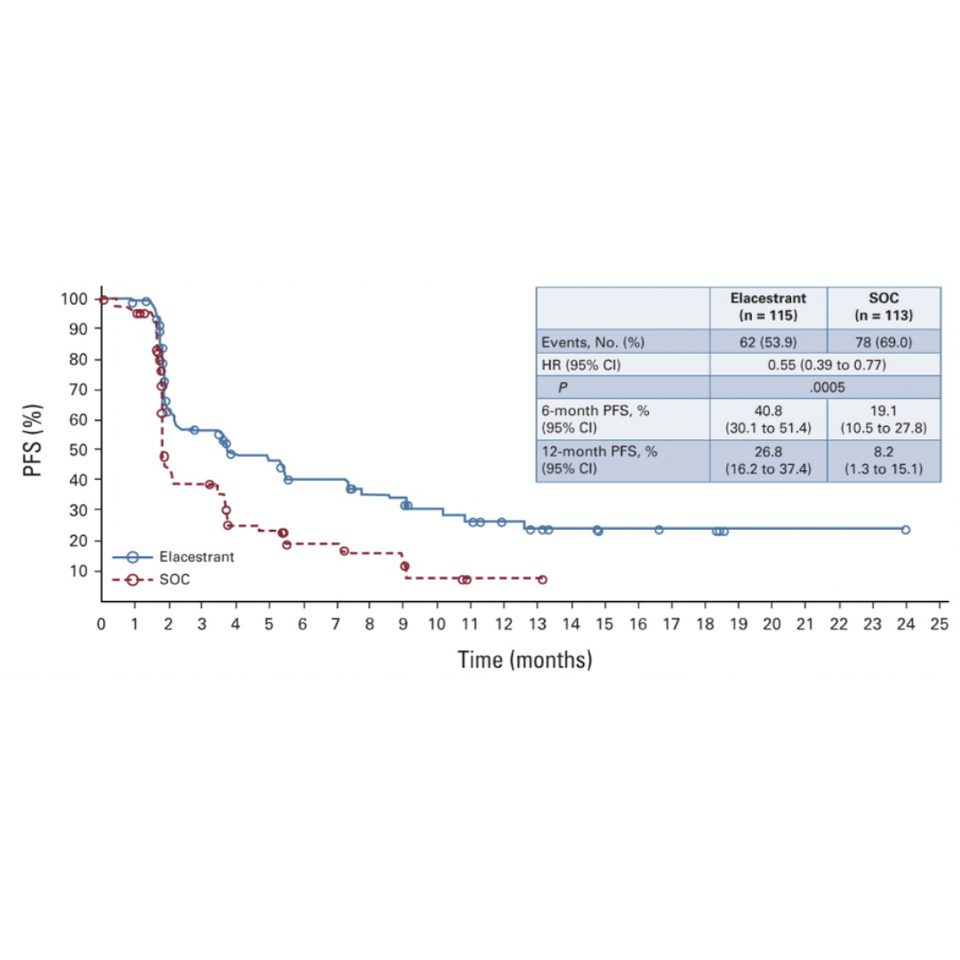
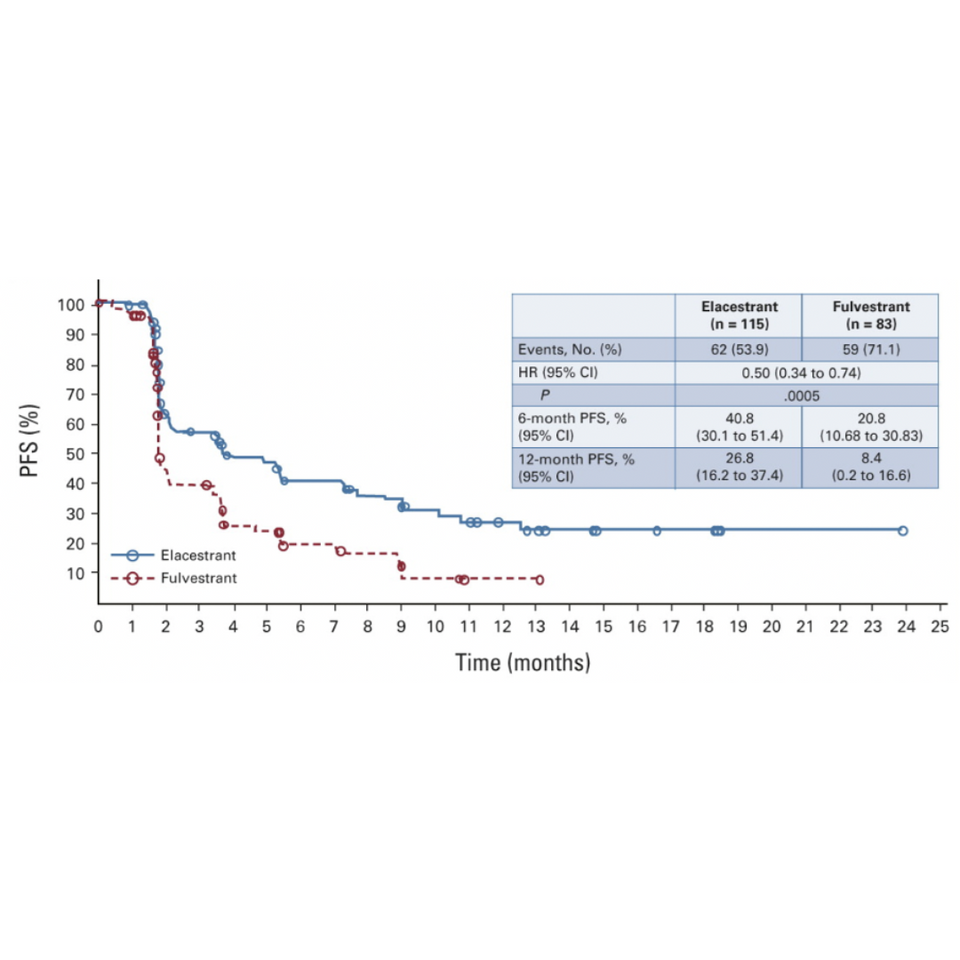
EMERALD – a phase III global randomized controlled trial was conducted to compare the efficacy and safety of elacestrant compared with SOC endocrine therapy in patients with ER-positive/HER2-negative advanced or metastatic breast cancer. The study had two primary endpoints, PFS in all patients and PFS in patients with detectable ESR1 mutations. 47.8% of patients in the study had detectable ESR1 mutation. [1]
Patients had progressed after first- or second-line treatment with a combination of endocrine therapy and a CDK4/6 inhibitor and the efficacy between arms in patients with detectable ESR1 mutation was compared. [1]
The 12-month rate of progression-free survival (PFS) was 26.8% with elacestrant vs 8.2% with the standard of care for patients with an ESR1 mutation (HR = 0.55; 95% CI, 0.39 to 0.77; P = .0005). [1]
In ESR1-mutated patients, 12-month PFS results of elacestrant compared with fulvestrant was 26.8% vs 8.4% respectively (HR=0.5; 95% CI, 0.34 to 0.74; P = .0005). [1]
Among patients with tumors harboring the ESR1 mutation, elacestrant was associated with a 45% reduction in the risk of progression or death compared with standard therapy. In this subgroup, the median PFS was 3.79 months with elacestrant and 1.87 months with standard endocrine therapy (HR, 0.54; p =.0005). [4]
See the full study publication here: https://pubmed.ncbi.nlm.nih.gov/35584336
Are you aware that ESR1 mutations lead to endocrine therapy resistant metastatic breast cancer?
What are your thoughts on novel therapies being developed that may be clinically important for these patients?
Click here to share your thoughts!
References
1. Bidard FC, et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol. 2022 Oct 1;40(28):3246-3256. doi: 10.1200/JCO.22.00338.
2. Pancholi, S., Simigdala, N., Ribas, R. et al. Elacestrant demonstrates strong anti-estrogenic activity in PDX models of estrogen-receptor positive endocrine-resistant and fulvestrant-resistant breast cancer. npj Breast Cancer 8, 125 (2022). doi.org/10.1038/s41523-022-00483-1
3. Zundelevich A, et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res 22, 16 (2020). doi.org/10.1186/s13058-020-1246-5
4. Jacobson A. Elacestrant Improves Progression-Free Survival After Endocrine Therapy for Estrogen Receptor-Positive Metastatic Breast Cancer. Oncologist. 2022 Mar 28;27(Suppl 1):S7-S8. doi: 10.1093/oncolo/oyac015.
This post is intended for US healthcare professionals and is supported by Stemline Therapeutics - part of the Menarini Group Company