COVID-19: Potential treatments
Potential Treatments
Due to the pace and scale of the outbreak, current investigation of potential therapies focuses on re-purposing existing approved drugs (1)
By 8th March there were 108 registered pharmacological COVID-19 trials (2) and there are currently over 1400 clinical trials looking for a treatment (3). Live trial updates and maps can be viewed on a tracker here.
On 22nd March WHO launched a global trial, SOLIDARITY, of the most promising 4 therapies, Remdesivir, Chloroquine or hydroxychloroquine, Ritonavir/Lopinavir combination, and Ritonavir/Lopinavir with Interferon-Beta. The trial is designed to be as simple as possible measuring only death or recovery and takes account of local drug availability. It is randomised but not blinded. WHO hope to reduce the timescale of the standard RCT by 80%. As of 3rd June, over 3.5 thousand patients have been enrolled in 35 countries (4)
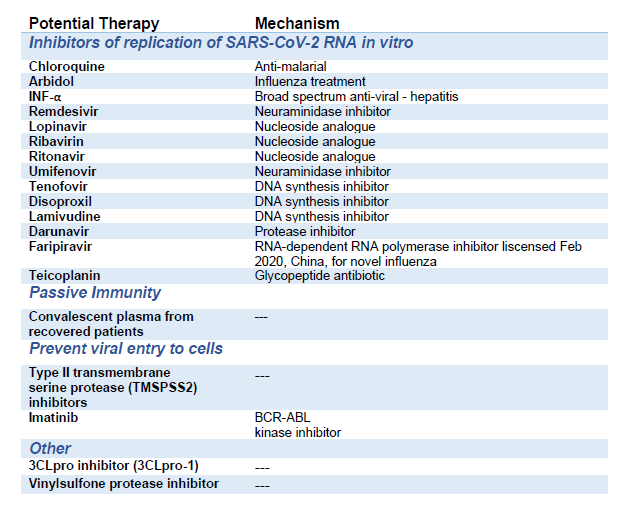
Although it is too early for any RCT data, the below potential therapies are amongst those being explored in the many trials (Table 1) (1,5,6,7,8,9,10). The majority of these potential treatments have pre-clinical data supporting their use.
Meta-analysis showed a significant reduction in mortality and viral load in SARS-CoV and MERS-CoV with use of passive immunity from convalescent serum of recovered patients (5) and further trials are ongoing into this potential therapy.
In case series from Wuhan, the majority of patients were prescribed anti-virals and empirical antibiotics (6). WHO recommends against empiric antibiotic cover in mild COVID-19, and in moderate COVID-19 unless there is suspicion of bacterial infection (particularly <5 years and elderly). WHO also recommends against use of antivirals for prevention or treatment of COVID-19 outside of clinical trials.
More recently the RECOVERY trial showed a significant reduction in deaths of COVID-19 patients who were either on oxygen or ventilation, and treated with daily Dexamethasone. You can read the full blog here.
References
1.Baron et. al. Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19? Int J Antimicrob Agents. 2020 Mar 13:105944. Availabile from: https://www.ncbi.nlm.nih.gov/pubmed/32179150 [Epub ahead of print, accessed 20.3.20]
2. Aronson J, Ferner R, DeVito N, Heneghan C. COVID-19 Registered Trials – and analysis. [Online] Available from: https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/ [Accessed 20.3.20]
3. Thorlund K et. al. Global Coronavirus COVID-19 Clinical Trial Tracker. [Online] Available from: https://www.covid-trials.org/ [Accessed 11.6.20]
4. World Health Organisation. "Solidarity" Clinical Trial for COVID-19 Treatments. [Online] Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [Accessed 11.6.20]
5. Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020 Mar 16;24(1):91. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32178711 [Accessed 20.3.20]
6. Mair-Jenkins J, Saavedra-Campos M, Kenneth Baillie J, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. 2014; Available from: https://academic.oup.com/jid/article-abstract/211/1/80/799341. [Accessed 20.3.20].
7. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020 Mar;55(3).Available from: https://www.sciencedirect.com/science/article/pii/S0924857920300674?via%3Dihub . [Epub ahead of print, accessed 20.3.20]
8. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020 Mar 10. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32173110. [Epub ahead of print, accessed 20.3.20]]
9. Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020 Mar 4;155.Available from: https://www.ncbi.nlm.nih.gov/pubmed/32145402. [Epub ahead of print, accessed 20.3.20]
10. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58-60. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32147628 [Accessed 20.3.20]
This page was written by Dr Rachel Coles, paediatric trainee and first published on 20th March 2020.
Updated on 24th March 2020 as WHO launched SOLIDARITY trial, and again on 11th June 2020.
Contact covid@medshr.net with questions, feedback and resources.
Loading Author...
Sign in or Register to comment