Emerging Therapies for COVID-19: Current vaccination programmes (1) - key issues

Open article published on MedShr: 4th November 2020
Last updated: 4th November 2020
Every effort has been made to ensure that the information contained in this article is accurate and current at time of publication, but if you notice an error please contact covid@medshr.net and we will aim to address this. Please find MedShr’s full terms of service here.
Background and key issues
Developing a safe and effective vaccine against SARS-CoV-2 is an urgent public health priority, in the context of the Coronavirus pandemic. Vaccination efficacy and safety protocols are usually laborious and detailed, and ordinarily take years to complete. Traditional vaccine development can commonly take 15 years or more with a lengthy discovery phase, in which vaccines are designed and exploratory preclinical experiments are conducted. Due to the urgency, these timelines have been shortened dramatically, with collaboration between big pharma, governmental bodies, academic research bodies and the public. As a consequence, vaccine development can be a loaded and politicised issue. A balance between expediting the availability of a vaccine to those who need it most, and an assurance that no corners are cut with respect to safety and efficacy checks is paramount [1].
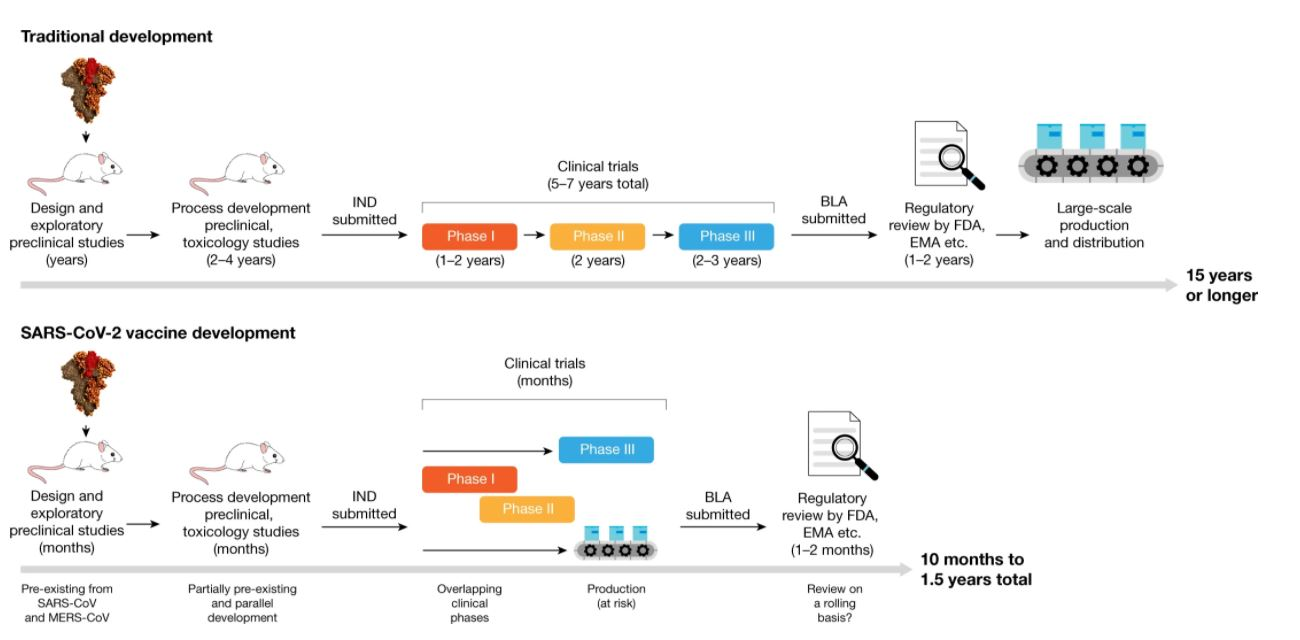
A recent review [2] details how traditional vaccine development varies from the much hastened SARS-CoV-2 vaccine development approach, and can be seen in Figure 1.
If and when a vaccine is approved, scarcity of supply in the early period of vaccination programmes enrolling will require the creation of frameworks (which include priority tiers) for the equitable distribution of Covid-19 vaccines.
Public confidence in science and in that underpinning vaccination is fragile. However, vaccination programs will succeed only if there is public belief that vaccines are safe and effective, and that policies underpinning the decisions on who gets vaccinated are backed by the most current evidence.
Vaccination types and programmes
The development of vaccines was first initiated when the genetic sequence of the virus became available in early January 2020, and has moved at an unprecedented speed since then. The first phase I trial started in March 2020, and there are currently more than 180 vaccines at various stages of development. Data from phase I and phase II trials are already available for several vaccine candidates, and many have moved into phase III trials. A recent review published in Nature estimates that these data are suggestive that safe and efficacious vaccines will be available within months, rather than years, as would normally take and had initially been feared [2].
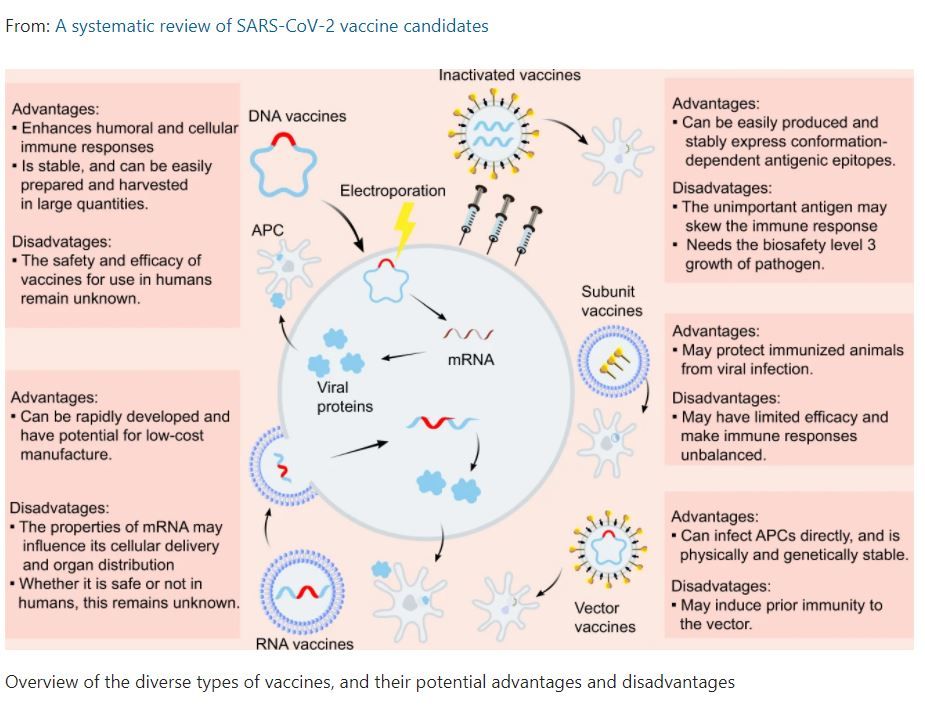
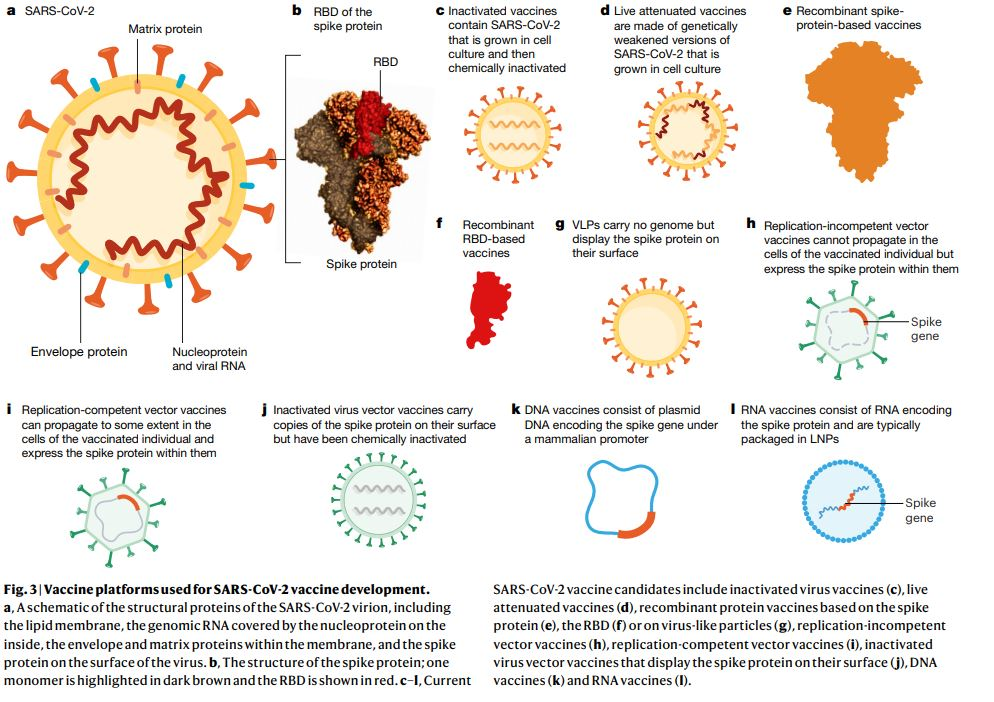
A recent review published on the key immunological approaches and targets to vaccine development is shown in figures 2 and 3 [2, 4]. A key approach to inducing antibody responses has been targeted against the SARS-CoV-2 spike protein, which facilitates binding to the angiotensin-converting–enzyme 2 (ACE 2) receptor and triggers virus–cell-membrane fusion, at which point there is a release of the viral genome into the cytoplasm [3].
Generally, vaccine development has been focused on an understanding of the virus, including its constituent proteins and structures. For example, the spike protein (S protein), main protease (Mpro), and RNA-dependent RNA polymerase (RdRp), have all been uncovered and produce important targets for the development of medications [4]. Further, an elucidation of the immune responses which SARS-CoV-2 induces have also been studied and constitute key targets for vaccine development. A summary of these different vaccination types, with their potential advantages and disadvantages taken from a recent review is seen in Figure 2 [4]. Other vaccine approaches are being currently pursued, including: inactivated vaccines, nucleic acid-based vaccines, and vector vaccines, all of which have already entered clinical trials [4].
Conclusion
As of the middle of September 2020, according to the World Health Organisation (WHO), 36 vaccine candidates were under clinical evaluation to treat COVID-19, and 146 candidate vaccines were in preclinical evaluation [4]. The World Health Organization (WHO) maintains a working document that includes most of the vaccines in development, and is available online [5].
With 10 SARS-CoV-2 vaccine candidates in phase III trials already, and a plethora of encouraging data from many candidate vaccines in earlier phase trials, there is some optimism. However, many unknowns remain, and phase III trials will need to demonstrate long term safety in large populations.
COVID-19 Research: Resources
There has been a vast amount of research published since the beginning of the pandemic. Trying to navigate this expanding catalogue of information to remain up-to-date with the latest developments is challenging, especially alongside clinical practice.
Below is a list of useful bodies and organisations who are making great efforts to categorise, evaluate and summarise this research to ensure optimal patient care globally; we hope you find this article useful and if you discover any other great resources please feel free to share these in the comments section below.
LitCovid: “LitCovid is a curated literature hub for tracking up-to-date scientific information about the 2019 novel Coronavirus... articles are updated daily and are further categorized by different research topics and geographic locations for improved access.”
National Institutes for Health: Coronavirus Disease 2019 (COVID-19) Treatment Guidelines
Continue the Discussion:
Join the COVID-19 discussion groups for healthcare professionals on MedShr:



References:
[1] Schwartz JL. Evaluating and Deploying Covid-19 Vaccines - The Importance of Transparency, Scientific Integrity, and Public Trust. N Engl J Med. 2020 Sep 23. doi: 10.1056/NEJMp2026393. Epub ahead of print. PMID: 32966716.
[2] Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516-527. doi: 10.1038/s41586-020-2798-3. Epub 2020 Sep 23. PMID: 32967006.
[3] Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271.e8-280.e8.
[4] Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020 Oct 13;5(1):237. doi: 10.1038/s41392-020-00352-y. PMID: 33051445; PMCID: PMC7551521.
[5] Draft Landscape of COVID-19 Candidate Vaccines. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (WHO, accessed 26 September 2020).
Article by Dr Adam Ali, MedShr Open Editorial Team, Medical Education Fellow
Loading Author...
Sign in or Register to comment