Emerging Therapies for COVID-19: Current vaccination programmes (2) - Development Challenges

Open article published on MedShr: 11th November 2020
Last updated: 11th November 2020
Every effort has been made to ensure that the information contained in this article is accurate and current at time of publication, but if you notice an error please contact covid@medshr.net and we will aim to address this. Please find MedShr’s full terms of service here.
Current vaccine trials (2): Challenges of vaccines
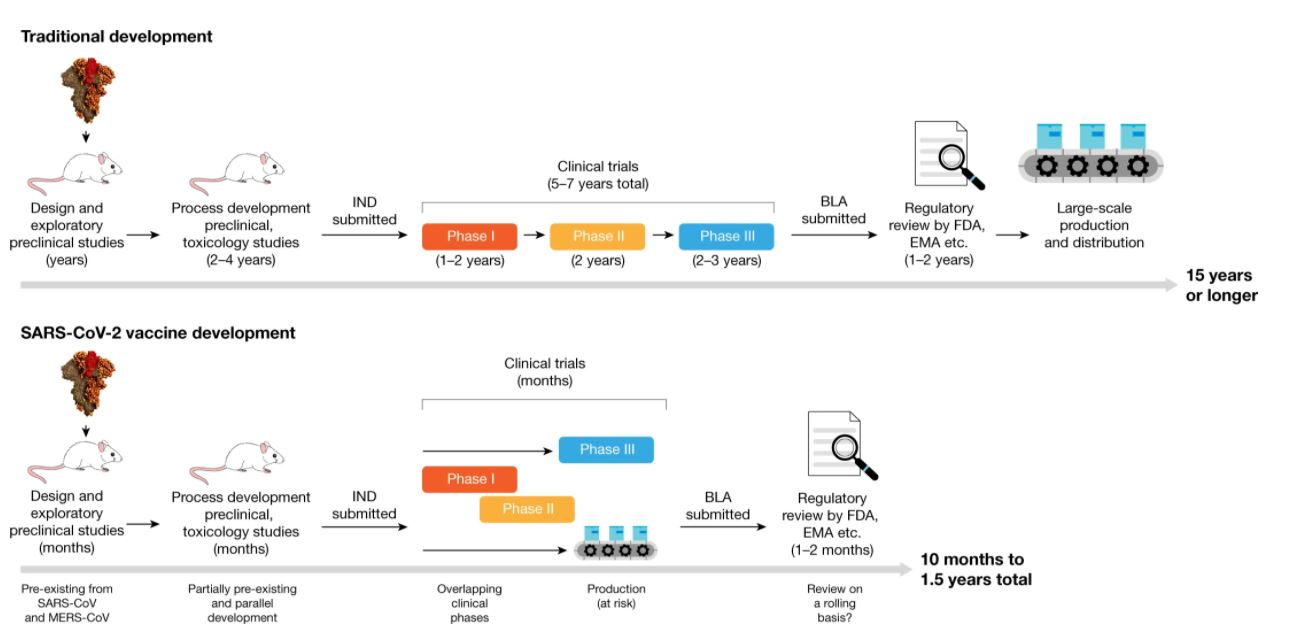
Developing a safe and effective vaccine against SARS-CoV-2 is an urgent public health priority. Traditional vaccine development commonly can take 15 years or more with a lengthy discovery phase, in which vaccines are designed and exploratory preclinical experiments are conducted (Figure 1). Due to the urgency, these timelines have been shortened dramatically, with collaboration between big pharma, governmental bodies, academic research bodies and the public [1].
There are currently 10 different SARS-CoV-2 vaccine candidates in phase III trials already, with encouraging results from many candidates in earlier phases. It is speculated that neutralizing antibodies could be a correlate of protection. However, this still needs to be verified in humans, and other factors—including cellular immune responses—might also have a protective role.
Routes
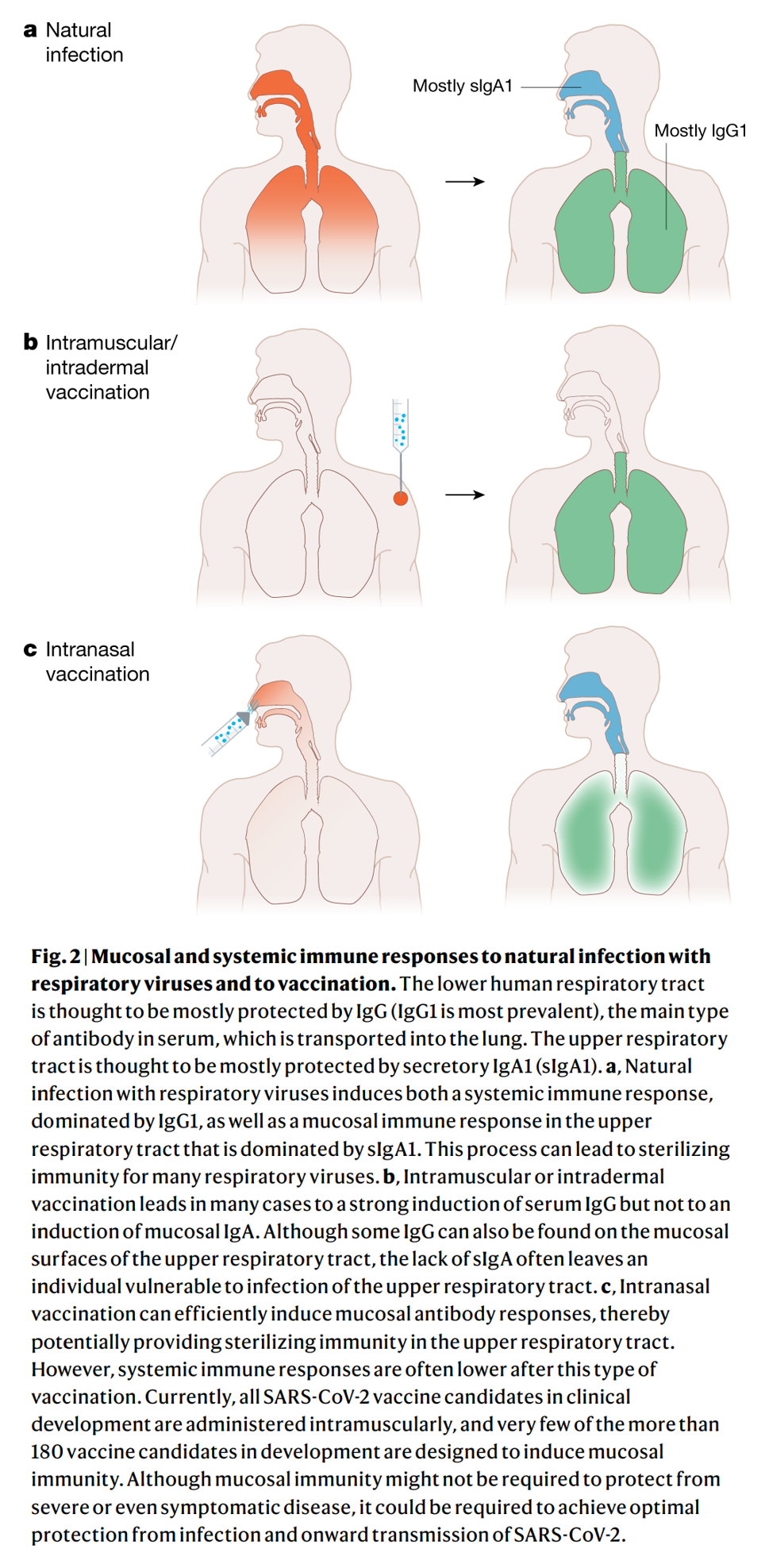
The current major vaccine candidates are all administered intramuscularly. This administration route induces strong IgG responses, which are thought to protect the lower respiratory tract. However, natural infection usually involves inoculation via the upper respiratory tract, which is thought to be protected mostly by secretory IgA responses. Small amounts of IgG can only be found in the upper respiratory tract in the case of very high serum titres. One concern, therefore, is that these current vaccines may fail to induce immunity for infections of the upper respiratory tract (Figure 2). This could lead to vaccines that would successfully protect from symptomatic disease, but might still enable transmission of the virus.
For this reason, a preferable vaccine would be one which could induce sterilizing immunity in the upper respiratory tract. A vaccine with intranasal route of administration would likely lead to a strong mucosal immune response, and also an IgG response. None of these are currently in clinical trials.
Vaccine-induced immunity
Another area of uncertainty surrounding new potential vaccinations is that it is unclear how long vaccine induced immunity will last. Natural infection is known to induce a ‘normal’ immune response, with a rise, peak and then reduction in the titre of antibodies over time from the point of inoculation. At this time, it is not known whether vaccine-induced immune responses are longer- or shorter-lived than immune responses induced by natural infection, and therefore, whether or not booster doses would be intermittently required to maintain immunity.
Vaccine tolerability in children and the elderly
Another area for exploration is how well older people will respond to the vaccine. This group also represents those who are most at risk from COVID-19 and developing serious complications. Several trials seem to show that the elderly respond less well than younger adults. Furthermore, alternations in vaccine formulation may make this another important parameter for predicting the induction of immunity. A pattern is seen in vaccines for protection from influenza virus, in which older patients require higher titres of antibodies for immunity than younger individuals. Therefore, a vaccine with higher reactogenicity that could induce a stronger antiviral response might be preferable in the elderly. Concerns about strong side effects means low-dose vaccines might be required for paediatric populations.
Rollout schedules and demand
The practicalities of distribution and rollout of potential vaccines are not yet clear, and will likely pose numerous challenges relating to effective and rapid manufacturing and delivery to patients. The first doses will likely be used to immunise high-risk groups and healthcare workers. Projections predict, assuming that two doses per person are needed, some 16 billion vaccine doses will be needed to meet the global demand. Physical commodities like the supply of syringes, glass vials and clinical equipment may form a bottleneck to vaccine availability. During scale-up, manufacturing and distribution of vaccine candidates, new challenges may arise, such as the need for frozen storage and distribution of mRNA and DNA vaccines, which becomes yet more difficult in low income countries.
In spite of these challenges, the variety of vaccines in advanced stages of development and the unprecedented speed of development are causes from optimism. At the time of writing, early reports of a candidate developed by Pfizer and BioNTech, tested on 43,500 people, with minimal safety concerns and some 90% effectivity are a staggering milestone reached remarkably quickly. Pfizer believes it will be able to supply 50 million doses by the end of this year, and around 1.3 billion by the end of 2021 [2], and the hope is that other candidates may be able to shortly follow suit.
COVID-19 Research: Resources
There has been a vast amount of research published since the beginning of the pandemic. Trying to navigate this expanding catalogue of information to remain up-to-date with the latest developments is challenging, especially alongside clinical practice.
Below is a list of useful bodies and organisations who are making great efforts to categorise, evaluate and summarise this research to ensure optimal patient care globally; we hope you find this article useful and if you discover any other great resources please feel free to share these in the comments section below.
LitCovid: “LitCovid is a curated literature hub for tracking up-to-date scientific information about the 2019 novel Coronavirus... articles are updated daily and are further categorized by different research topics and geographic locations for improved access.”
National Institutes for Health: Coronavirus Disease 2019 (COVID-19) Treatment Guidelines
Continue the Discussion:
Join the COVID-19 discussion groups for healthcare professionals on MedShr:



References:
[1] Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516-527. doi: 10.1038/s41586-020-2798-3. Epub 2020 Sep 23. PMID: 32967006.
[2] Accessed online at https://www.bbc.co.uk/news/health-54873105
Article by Dr Adam Ali, MedShr Open Editorial Team, Medical Education Fellow
Loading Author...
Sign in or Register to comment