World Malaria Day 2023

Malaria remains a significant global health issue, particularly in sub-Saharan Africa, which bears the brunt of the disease.
At MedShr we aim to promote awareness and deliver education around the disease. By sharing our experiences and knowledge, we can work together to improve patient outcomes and reduce the impact of this disease.
In this article, we will explore the latest information regarding malaria and its global impact, the challenges around treatment resistance, and the progress made in developing vaccines to combat this devastating disease.
Current Landscape: Malaria Deaths and Effects Globally
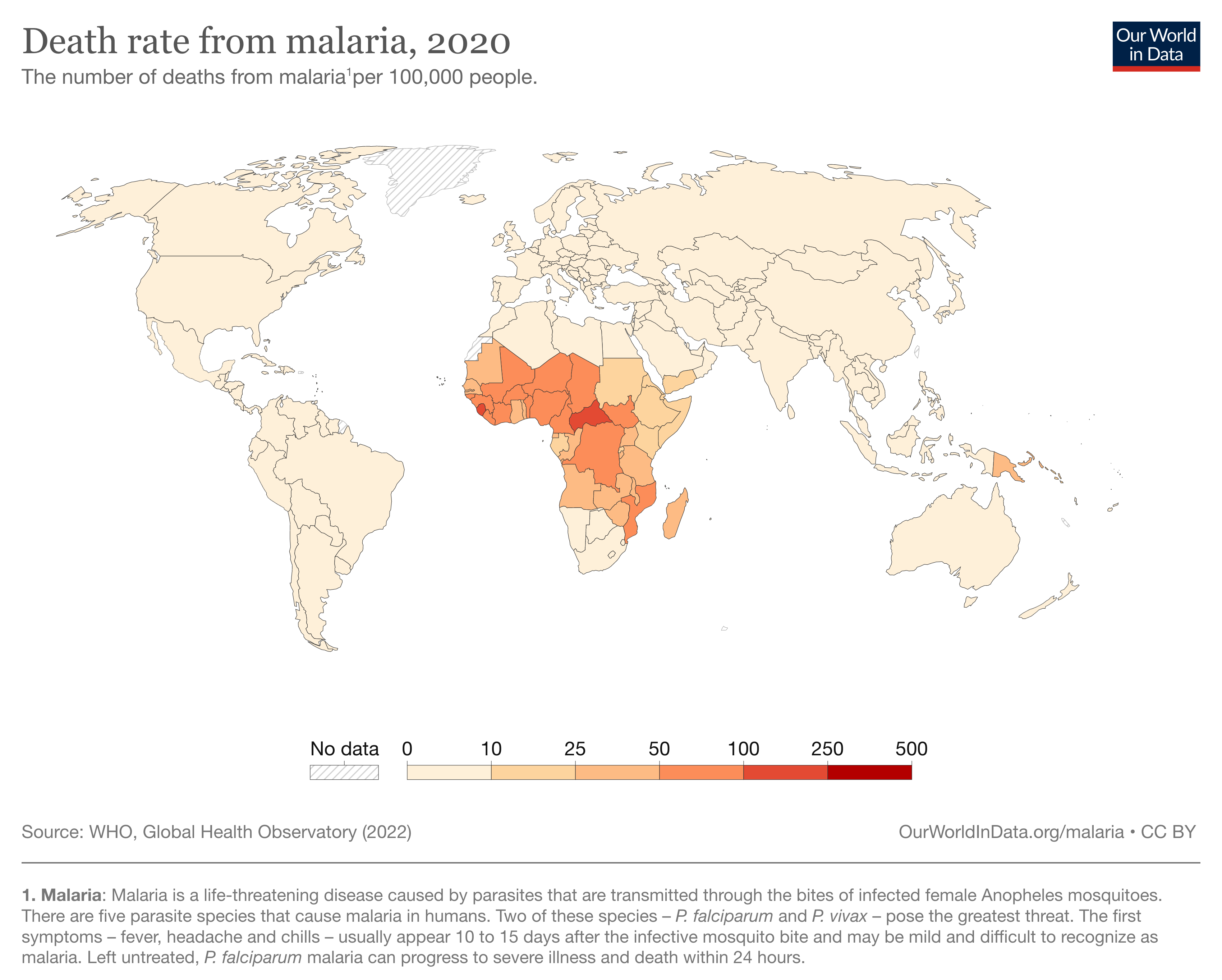
According to the World Health Organization (WHO), in 2021, there were an estimated 241 million cases of malaria worldwide, resulting in approximately 627,000 deaths [1]. Most of these cases (95%) and deaths (96%) occurred in sub-Saharan Africa, with young children and pregnant women being the most vulnerable [1].
Malaria not only has a severe impact on public health but also carries significant social and economic burdens. The disease affects productivity, leading to income losses and reduced economic growth. It is estimated that malaria costs Africa $12 billion per year in direct losses, and an additional $16 billion in indirect costs such as lost productivity [2].
Treatment Resistance: A Growing Concern
Artemisinin-based combination therapies (ACTs) have been the cornerstone of malaria treatment since the early 2000s. However, there is growing concern about the emergence and spread of parasite resistance to these drugs [3]. The rise of drug-resistant malaria parasites poses a significant threat to global efforts to control and eliminate the disease.
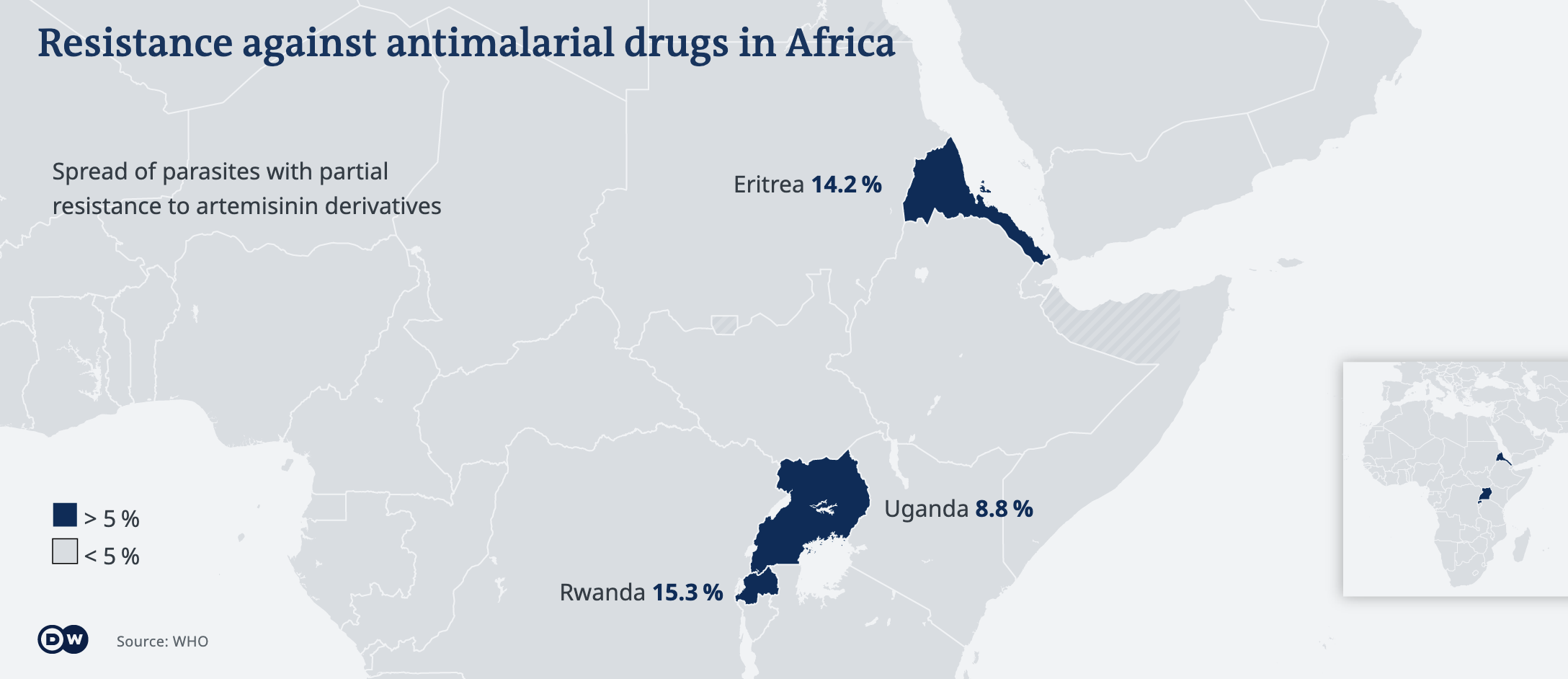
Recent studies have confirmed that malaria parasites resistant to artemisinin have emerged in Rwanda, Uganda and the Horn of Africa though experts believe that the problem is likely more widespread. Artemisinin partial resistance increases the risk of de novo emergence of partner drug resistance, and importantly, the spread of parasites less sensitive to the partner drugs, which can result in clinical treatment failures. Given the heavy reliance on ACTs in Africa, and the risk resistance poses on the management of malaria in the future, the threat of drug resistance must be addressed urgently.
Resistance to previous generations of antimalarial drugs, such as chloroquine and sulfadoxine-pyrimethamine, has led to increased morbidity and mortality, particularly in Africa [4]. It is crucial to monitor and manage drug resistance to prevent a similar scenario with ACTs. This includes improving surveillance systems, promoting rational drug use, and investing in research and development for new antimalarial drugs [3].
Quickly diversifying ACT markets in countries to reduce drug pressure is a key approach recommended by the WHO in their strategy to respond to antimalarial drug resistance in Africa. In the strategy, the WHO also calls for research to close evidence gaps on innovative approaches (e.g. multiple first line ACTs) to better use current tools as well as research on how these approaches might address drivers of resistance. [3]
Vaccine Development: A Ray of Hope
The development of an effective malaria vaccine has been a longstanding goal in the fight against the disease. In October 2021, the WHO endorsed the use of the RTS,S/AS01 (brand name Mosquirix) malaria vaccine for children in sub-Saharan Africa and other high-risk areas [5]. This vaccine has shown a reasonable efficacy of about 30% in reducing severe malaria cases in young children [5].
The approval of Mosquirix marks a significant milestone, but it is not a silver bullet. The vaccine's moderate efficacy means it must be used alongside existing malaria control measures, such as insecticide-treated bed nets and prompt diagnosis and treatment [5].
Research into next-generation malaria vaccines is ongoing.
Image courtesy of Gavi - White Rhino Films-Lameck Orina
The R21/Matrix-M malaria vaccine, developed by the University of Oxford's and manufactured and scaled up by the Serum Institute of India, has shown promising results in a phase 2b clinical trial conducted in Burkina Faso. The trial included 450 children between the ages of 5 and 17 months, and the vaccine demonstrated 77% efficacy over a 12-month follow-up period, with no serious safety concerns reported [6]. This level of efficacy surpasses the World Health Organization's goal of a malaria vaccine with at least 75% efficacy by 2030 [7]. The R21 vaccine is currently undergoing further testing in a larger phase 3 trial across several African countries to confirm its efficacy and safety [8].
In April 2023, Ghana became the first country to approve the highly anticipated R21 malaria vaccine that could save millions from the mosquito-borne disease.

At MedShr we are building a network of health care professionals interested in malaria and its global impact. We aim to promote awareness and deliver education around the disease.
Join our Malaria Global Education Network - through sharing your experiences of managing patients with suspected malaria and your practical solutions to overcome barriers to prophylaxis, screening and treatment, we believe this network will help to improve the lives of patients globally and ultimately save lives.
All MedShr posts in this group are intended for HCPs and are part of an educational program funded by Novartis.
References
[1] World Health Organization. (2021). World Malaria Report 2021. Retrieved from https://www.who.int/publications/i/item/9789240040499
[2] Gallup, J. L., & Sachs, J. D. (2001). The economic burden of malaria. The American Journal of Tropical Medicine and Hygiene, 64(1_suppl), 85-96. https://doi.org/10.4269/ajtmh.2001.64.85
[3] World Health Organization. (2021). Artemisinin resistance and artemisinin-based combination therapy efficacy. Retrieved from https://www.who.int/malaria/areas/drug_resistance/overview/en/
[4] Fidock, D. A., Nomura, T., & Wellems, T. E. (1998). Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Molecular Pharmacology, 54(6), 1140-1147. https://doi.org/10.1124/mol.54.6.1140
[5] World Health Organization. (2021). Malaria vaccine: WHO position paper, January 2021. Retrieved from https://www.who.int/publications/i/item/WHO-WER-9702
[6] Datoo, M. S., Natama, M. H., Somé, A., Traoré, O., Rouamba, T., Bellamy, D., Yameogo, P., Valia, D., Tegneri, M., Ouedraogo, F., & Espoir B. P. (2021). Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. The Lancet, 397(10287), 1809-1818. https://doi.org/10.1016/S0140-6736(21)00943-0
[7] World Health Organization. (2013). Malaria Vaccine Technology Roadmap. Retrieved from https://www.who.int/immunization/topics/malaria/vaccine_roadmap/TRM_update_nov13.pdf?ua=1
[8] University of Oxford. (2021). Oxford malaria vaccine proves highly effective in Burkina Faso trial. Retrieved from https://www.research.ox.ac.uk/Article/2021-04-23-Oxford-malaria-vaccine-proves-highly-effective-in-Burkina-Faso-trial
Loading Author...
Sign in or Register to comment