COVID-19 Prevention: Vaccine Development

What is the timeline on a Coronavirus vaccine? (1)
-On 11th Jan 2020 the genetic sequence for SARS-CoV-2 becomes available
-On 16th March 2020 the first human clinical tests of a candidate vaccine begin (Clinical development - Phase 1)
How long does it usually take to develop vaccines? (2)
Traditionally, the process can take several years if not exceeding a decade
To develop a vaccine at pace requires parallel working where the different steps are carried out at once
The infographic below demonstrates the attrition and cost of vaccine development as well as the usual timeline. Comparing this to the SARS-CoV-2 timeline above shows the accelerated nature of SARS-CoV-2 vaccine development
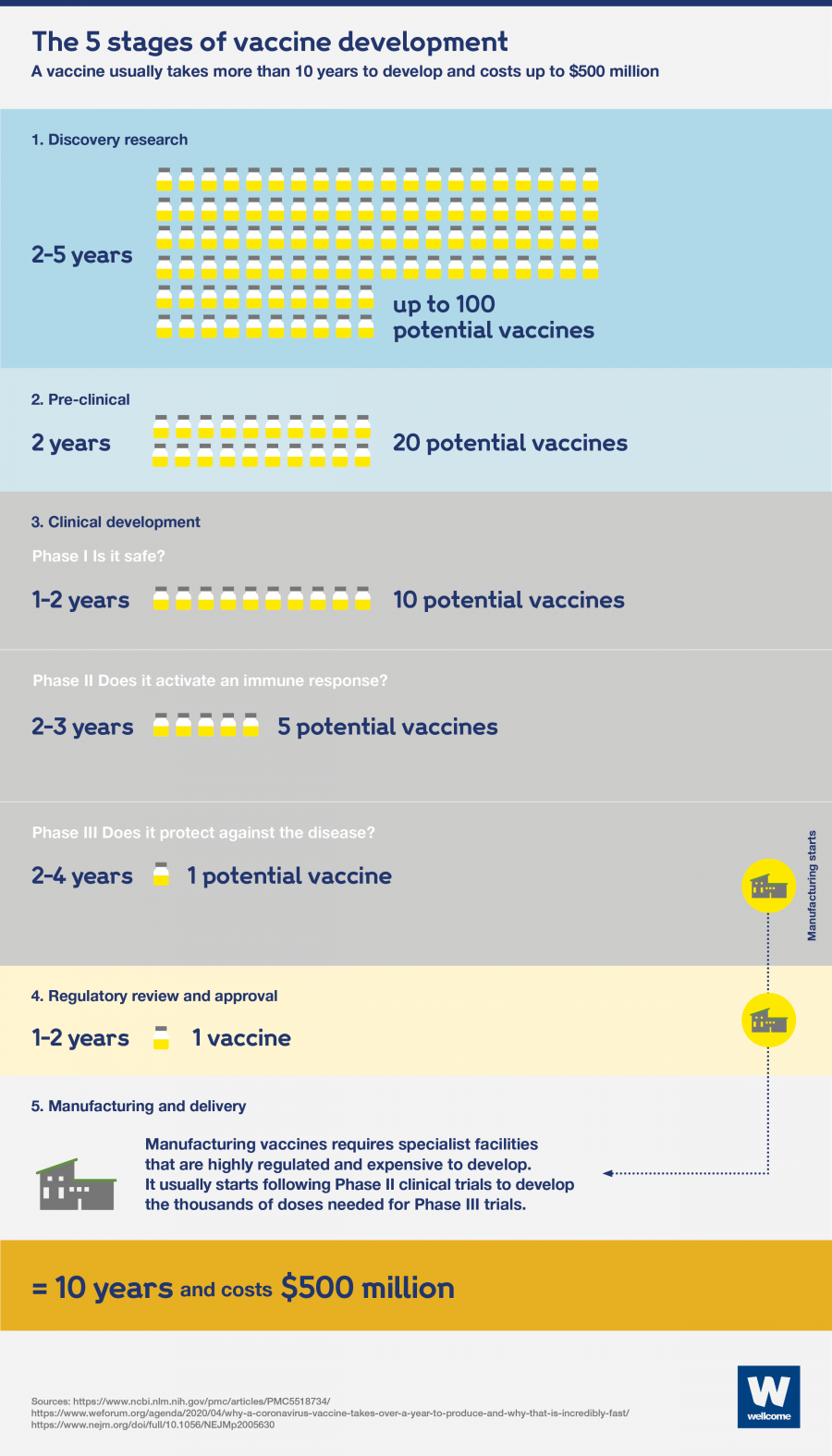
Source: Wellcome
How many COVID-19 vaccines are being developed? (1)
-There are around 115 candidate vaccines (as of April 2020)
-Most are in pre-clinical stages
-At least 5 are known to be in clinical development
Who is developing COVID-19 vaccines? (1)
-Most (72%) are being developed by private companies and industry
-Public sector, academic and non-profit vaccine development makes up 28%
Where are Coronavirus vaccines being developed? (1)
-46% of the developers are based in North America
-18% are in China
-18% are in rest of Asia
-18% are in Europe
What does this mean for Africa and the Middle East?
The data above show low proportions of vaccine development in Africa and Middle East, which is reflected in the signatories of the International Collaboration on COVID-19 Vaccine Development, co-ordinated by WHO (3)
Vaccine development must include countries from across the world in order to ensure all populations will be protected (2)
Vaccine manufacturing must also include representation from countries across the world so that access is equitable (2)
Capacity and regulatory frameworks for vaccine development exist, which could be utilised by governments and the international community (2)
What are the differences between the COVID-19 vaccine strategies used? (4)
-Whole virus vaccines: have intrinsic immunogenicity but often require additional thorough testing to ensure they are safe. Examples of this vaccine type include live attenuated and inactivated vaccines.
-Sub-unit vaccines: can minimise host immunopotentiation but rely on an immune response being triggered against S-spike protein (preventing viral entry to cells).
-Nucleic acid vaccines: have undergone recent new adjustments to the technology which show promise, however no previous vaccine has been successful using this method. Examples include DNA vaccines.
More detailed information on vaccine types is available here.
The chart below demonstrates the wide array of techniques being explored in the search for a Coronavirus vaccine:
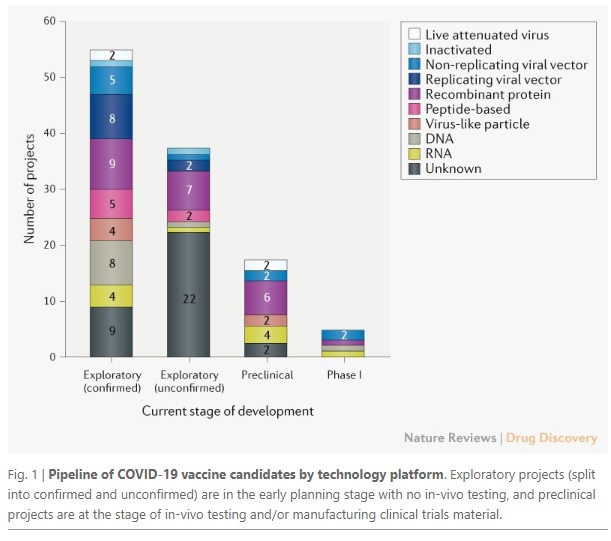
Source: Nature
What do we know about the SARS-CoV-2 vaccines in Phase 1 trials? (5)
There is a US trial by Moderna of an mRNA vaccine encoding S-protein estimated to include 105 participants and to complete 20th September 2020 (primary conclusions).
There is a Chinese trial by CanSino Biologics of an adenovirus vector expressing S-protein, estimated to include 108 participants and to complete 30th December 2020 (primary conclusions).
There is a US trial by Inovio Pharmaceuticals of a DNA plasmid encoding S-protein estimated to include 40 participants and to complete April 2021 (primary conclusions).
There are two Chinese trials by Shenzhen Geno-Immune Medical Institute using lentiviral vectors (aAPC, DCs), both estimated to include 100 participants and to complete July 2023 (primary conclusions).
There is a UK based trial in Oxford by Oxford University and Imperial College London using an adenovirus vaccine vector and the SARS-CoV-2 spike protein, estimated to include over a thousand participants and to complete in September 2020 (primary conclusions).
Written by Dr Rachel Coles, Education Fellow, 4th May 2020.
1. Thanh Le T et. al. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery. 19, 305-306 (2020). doi: 10.1038/d41573-020-00073-5
2. Wellcome. How can we develop a COVID-19 vaccine quickly? [Online] Available from: https://wellcome.ac.uk/news/how-can-we-develop-covid-19-vaccine-quickly [Accessed 5.5.20]
3. World Health Organisation. Public statement for collaboration on COVID-19 vaccine development. [Online] Available from: https://www.who.int/news-room/detail/13-04-2020-public-statement-for-collaboration-on-covid-19-vaccine-development [Accessed 5.5.20]
4. National Institute of Allergy and Infectious Diseases. Vaccine Types. [Online] Available from: https://www.niaid.nih.gov/research/vaccine-types [Accessed 5.5.20]
5. U.S National Library of Medicine Clinicaltrials.gov [Online]. Available from: https://clinicaltrials.gov/ [Accessed 5.5.20]
Loading Author...
Sign in or Register to comment